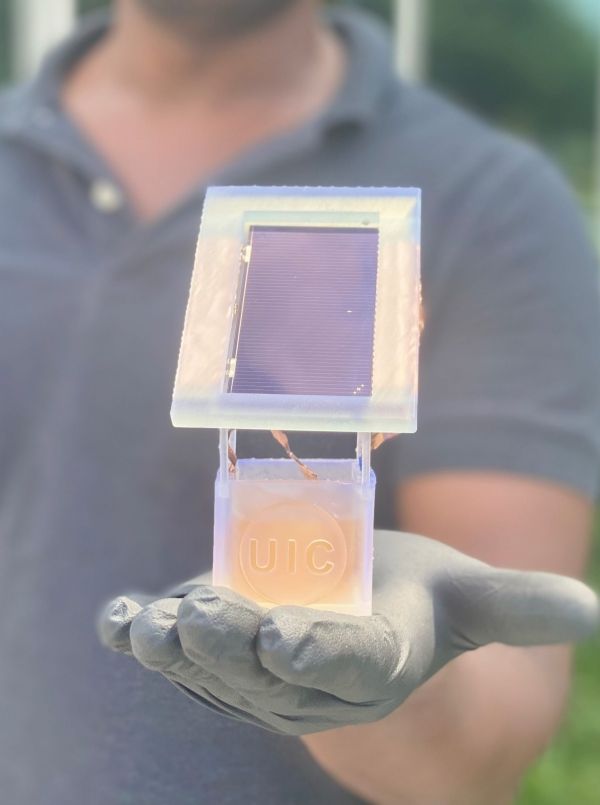
Researchers at UIC are developing a long-term electrochemical device that combines a solar cell with a liquid solution in a well. Nitrates via wastewater mostly in liquid solution are transformed to ammonia during charging. Researchers at the University of Illinois Chicago recently developed an electrochemical solar energy reaction which not only uses wastewater to manufacture ammonia – the world’s second most-produced chemical – but also has a solar energy efficiency that is ten times higher than almost any comparable technology. This technique and approach offer a lot of promise for on-demand fertilizer synthesis, and they may have a big influence here on agriculture as well as energy industries in both rich and developing nations, as well as try to decrease greenhouse gas emissions from fossil fuels.
Researchers devised a novel technique that relies on nitrate, among the most prevalent pollutants in groundwater, to provide nitrogen and sunshine to electrify the process. With almost few hydrogen gas side reactions, the system generates approximately 100% ammonia. The process uses no fossil fuels as well as emits zero carbon dioxide from the atmosphere as well as other greenhouse gas emissions, and yet it uses solar electricity to achieve exceptional sun-to-fuel effectiveness, as well as STF, around 11%, which is ten times higher than any other current ammonia production system (about 1 percent STF).
Given that the usual method of applying fertilizer is to simply spray it on the soil then wait for rainfall to wash it into the soil, the wastewater nitrates are most likely due to runoff by fields overdosed with fertilizer. These nitrates (as well as other soluble substances) would undoubtedly be washed into the waterways as a result. Ammonia is a nitrogen-based molecule with three hydrogen atoms that are used in fertilizers and a variety of industrial goods including plastics and medicines. To break the tight bonds among nitrogen atoms such that they could connect with hydrogen, current techniques of producing ammonia using nitrogen require large quantities of heat, which is provided by the burning of fossil fuels. This centuries-old process is responsible for a large percentage of global greenhouse gas emissions, which are a major contributor to climate change.
Also, there is a method (not very well in use) of injecting fertilizer down into the soil, which reduces the likelihood of it being washed away, allowing you to use considerably less fertilizer to achieve the same results while also avoiding groundwater contamination. That seemed to me to be a rather appropriate place to do – it saves money while also reducing issues. The disadvantage is that you’ll have to alter the ways people do stuff and they’ll have to invest in new equipment, so there will be a pay-back period. Nonetheless, it does address the issue with groundwater contamination that is well worth the effort for society.
The new technique uses a cobalt catalyst, which researchers explain in their article, “Solar-Driven Electrochemical Production of Ammonia utilizing Nitrate with 11 percent Solar-to-Fuel Efficiency under Ambient Conditions,” along with the novel procedure. Researchers from the University of Illinois at Chicago explain their long-term electrochemical device for converting wastewater nitrate to ammonia.
The researchers initially used computational theory to determine which metal would function best as a catalyst. After finding cobalt to use these simulations, the team tested with metal then attempted a variety of methods to improve the reaction’s activity. The researchers discovered that a rougher cobalt surface is being produced by oxidation was optimal for producing a selective reaction, in which virtually every nitrate molecule is transformed into ammonia.
We must additionally eliminate the pollutant from soil and groundwater if we use wastewater nitrate. This implies that, over time, the procedure can help rectify industrial waste as well as water run-off while also rebalancing the nitrogen cycle, which is especially important in rural regions that may face economic challenges or are in danger from excessive nitrate exposure.
The main concern with this pyrolysis of waste Nitrates to Ammonia is that it would very certainly be utilized to make additional Nitrates if done on a large scale, is just what percentage of Nitrates would be required in the input. After all, if a concentrated solution is required, you’ve already completed much of the effort required to simply retrieve the Nitrates and distribute them on the ground once more. If it can work at a concentration comparable to wastewater, you’ll be creating a small proportion of Ammonia that will dissolve in water, necessitating the use of energy to distill the material out. In any case, even if the chemical energy we utilize is solar and thus free, you’ll need to invest more energy to produce a usable product. As a result, I believe this procedure is not cost-effective, even though it presumably accomplishes just what says on the label.
This technique might be beneficial for eliminating Nitrates from groundwater by simply letting the generated Ammonia evaporate through into air inside a shallow settling pond. As a result, a component of the system is utilized to purify drinking water before it is distributed thru the pipe network. However, it may be preferable to prevent Nitrates from entering the water, however even if we accomplished that, there would still be a buildup to deal with, so it may be a helpful addition to water purification for a few decades.
The process rate will fluctuate with the climate as well as the season because it is solar-powered; hence the efficiency of this two-pound system would be significantly lower during gloomy winter days. Thus, to just have something which is always adequate, you’ll have to overprovision the system by the factor of maybe ten times how much you’d require if you have got a known efficacy that operated 24 hours a day, seven days a week.
Chemistry is a really interesting subject to learn about, especially with all the developments seen in the industry as time goes on. As one of the core sciences taught in the education system, Chemistry has been able to equip people with knowledge and skills to better understand the functions that underpin society, such as technology, medicine, food, and much more. Matter makes up everything that we know, and are the building blocks of the world. You can learn more about this incredible science by exploring the Chemistry Database.
Sources:














