
The chemical formula h-BN stands for hexagonal boron nitride, a chemically and thermally resistant refractory compound of boron and nitrogen. It appears in a variety of crystalline forms that are isoelectronic to a carbon lattice with a comparable structure. Among the BN polymorphs, the hexagonal form equivalent to graphite is the most solid and soft and is hence utilized as a lubricant and cosmetic component.
The iron man of 2D materials, hexagonal boron nitride, is so resistant to shattering that it contradicts a millennium theoretical characterization engineers still use to measure toughness. Jun Lou of Rice University explained that what they discovered in hexagonal boron nitride is extraordinary and no one anticipated seeing this in 2D materials. That is what makes it so thrilling.
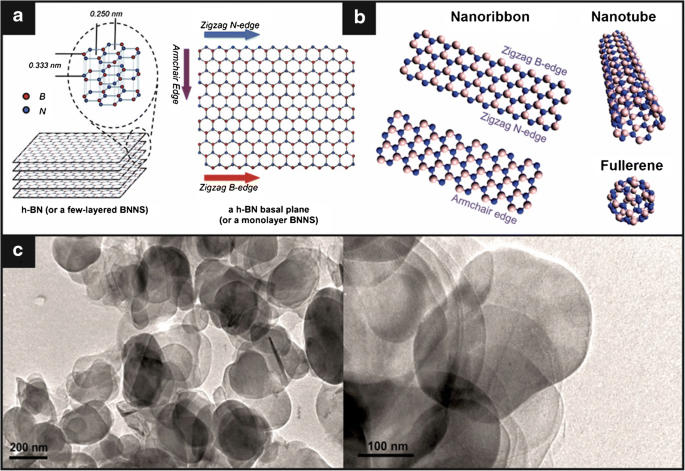
cr:https://link.springer.com/article/10.1007/s00216-020-03068-8/figures/2
Relationship Between Hexagonal Boron Nitride And Graphene
Lou compares the fracture toughness of h-BN to that of its more well-known relative graphene to demonstrate the significance of the finding. Graphene and h-BN are structurally nearly identical. Atoms are arranged in a flat lattice of connected hexagons in each of them. All of the atoms in graphene are carbon, but each hexagon in h-BN has three nitrogen and three boron atoms.
Graphene has the strongest carbon-carbon bonds in nature, making it the hardest material on the planet. However, there is a catch. Graphene’s performance can shift from amazing to poor if even a few atoms are out of place. And no material is defect-free in the real world, according to Lou, which is why fracture toughness — or resistance to crack propagation — is so critical in engineering: It specifies the maximum amount of abuse a real-world substance may take before failing.
Graphene, in a nutshell, is fragile. A.A. Griffith, a British engineer, characterized the failure of brittle materials in seminal theoretical research on fracture mechanics published in 1921. Griffith’s research established a link between the size of a crack in a material and the force necessary to develop it. Griffith’s time-tested criterion could be explained by the fracture toughness of graphene, according to Lou’s 2014 study. Given its structural resemblance to graphene, h-BN was expected to be fragile as well.
That isn’t correct. The fracture resistance of hexagonal boron nitride is nearly ten times that of graphene, and the performance of h-BN in fracture testing was so unforeseen that it violated Griffith’s formula. Over 1,000 hours of testing in Lou’s lab at Rice University and similarly arduous theoretical work led by co-corresponding author Huajian Gao at Nanyang Technological University (NTU) in Singapore were required to show exactly how it operated and why.
What excited Gao about this research is that it reveals an underlying toughening mechanism in a material that is supposed to be brittle. Even Griffith couldn’t have predicted such significantly divergent fracture behaviors in two brittle materials with identical atomic structures.
The dramatically divergent material behaviors were traced back to minor asymmetries caused by h-BN possessing two components instead of one, according to Lou, Gao, and colleagues. Boron and nitrogen are completely different, so even though there is a hexagon, because of the asymmetric arrangement, it is not the same as the carbon hexagon (in graphene). This fact was stated by Lou.
The theoretical description is difficult, according to Lou, but the result is that cracks in h-BN tend to branch and turn. The point of the crack in graphene passes straight through the substance, like a zipper, opening bonds. However, the asymmetry of the lattice in h-BN causes a “bifurcation” where branches can develop.
“If the crack is branched, that means it’s turning,” Lou explained. According to him, If you have a turning crack, it effectively costs you more energy to push it further. So you’ve successfully toughened your material by making crack propagation considerably more difficult. His colleague, Goa explained that the intrinsic lattice asymmetry endows h-BN with a permanent tendency for a moving crack to branches off its path, like a skier who has lost her or his ability to maintain a balanced posture to move straight forward.
Hexagonal boron nitride Application
Heat resistance, dielectric qualities, and chemical stability enable hexagonal boron nitride to act as both an insulating layer and a supporting foundation between electronic components, making it a significant material for 2D electronics and other applications. The remarkable toughness of h-BN, according to Lou, could make it an attractive alternative for adding tear resistance to flexible electronics constructed of fragile 2D materials.
Lou explained that you need to make sure the material is mechanically resilient when you bend it around for this type of configuration. According to him, the fact that h-BN is fracture-resistant is fantastic news for the 2D electronic field as it means this material may be used as a very useful protective coating. The discoveries could potentially indicate a new way to make tough mechanical metamaterials using designed structural asymmetry.
Sources:
- Jade Boyd 713-348-6778 [email protected], Rice University
- https://phys.org/news/2021-06-hexagonal-boron-nitride-remarkable-toughness.html
- Intrinsic toughening and stable crack propagation in hexagonal boron nitride, Nature (2021). DOI: 10.1038/s41586-021-03488-1 , www.nature.com/articles/s41586-021-03488-1














