Credit: University of Cambridge
Scientists built a small camera with a ‘molecular glue,’ which enables them to view chemical reactions in real-time. A team at Cambridge University developed the mechanism that integrates gold nanoparticles and small nanocrystals called quantum dots, with a molecular glue known as cucurbituril (CB). When the components are put to the water with the molecule that will be examined, they auto assemble in seconds into a strong and stable tool for detecting chemical reactions in real-time.
The camera collects lights in semiconductors, causing processes of electron transfer, such as photosynthesis, which can be examined using spectroscopic methods and gold nanoparticles. The camera was used to view chemical species which had before been theorized but not witnessed directly. The platform might be used to examine a broad array of molecules for several possible uses, including photocatalysis enhancement and renewable energy photovoltaics.
In self-limiting processes, nature regulates the assembling of complex structures on the molecular scale. However, it is frequently time-consuming, costly, and sophisticated methods to imitate these processes in the laboratory.
Professor Oren Scherman from Cambridge’s Yusuf Hamied department of chemistry who led research explained that in order to build new materials with exceptional qualities, we frequently combine diverse chemical species to create a hybrid material with the features we aim to achieve. However, he mentioned that it is challenging to make these hybrid nanostructures and you sometimes result in unregulated development and unstable materials.
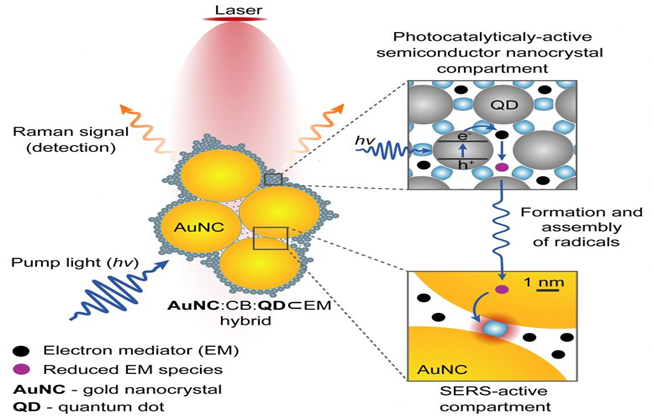
Scherman and his partners at the Cavendish Laboratory and University College London, Cambridge, have created a new technique of cucurbituril, a molecular glue that is extremely active with both gold nanoparticles and semiconductor quantum dots. The scientists utilized small semiconductor nanocrystals to regulate the assembling of bigger nanoparticles utilizing an interfacial composition. The technique results in lasting and permanent hybrid materials interacting with light. The camera is utilized to monitor catalytic photography and monitor light-induced electron transfer.
Dr. Kamil Sokołowski, the first researcher, from the Chemistry Department explained that they have been surprised at the strength of this new instrument, as the assembly is easy. The scientists mixed water at room temperature to build their nano-camera with the particular elements they intended to examine. In the past, in the absence of quantum dots, when gold nanoparticles were combined with molecular glue, elements were aggregated without restriction and fell out of a solution. But in line with the study method, the quantum dots regulate the assemblage of these nanostructures, to regulate and restrict the shape and size of the semiconductor-metal hybrids Moreover, they remain steady over weeks.
Co-author Dr. Jade McCune, from the Chemistry Department, stated that it was astonishing that this self-limiting property was not something that we anticipated to find. According to him, It could be discovered that adding another nano part of the aggregate of one nanoparticular component was controlled.
The team employed spectroscopy to monitor chemical reactions in real-time while scientists combined the substances. They were able to view the production of radical species — molecules with an unpaired electron – as well as the results of their assembly, such as sigma dimeric viologen species, which are formed when two radicals establish a reversible carbon-carbon bond. The second species had been speculated about but never seen.
Scherman, who is also the Director of the Melville Laboratory, remarked that people spent their entire lifetimes getting fragments of matter to join together in a regulated manner. According to him, this system will open up a large variety of processes, including numerous materials and chemistries that are critical for developing sustainable technology. The entire capability of nanocrystals in semi-conductors and plasmons may now be investigated and photochemical reactions can concurrently be generated and observed.
This system is a really large toolbox that takes into account the number of component blocks of metals and semiconductors that can be combined using the same chemical system, offering many new opportunities for imaging and sensing chemical reactions through snapshots of the controlled chemical systems. For scientists, simplicity means that complex, costly approaches are not required anymore to produce the same results.
Researchers in the Scherman lab is presently attempting to improve these hybrids so that they can be used to create artificial photosynthetic systems and (photo)catalysis where electron-transfer processes can be viewed in real-time. Electrode interfaces and mechanisms of carbon-carbon bond formation for battery applications are also being studied by researchers.
Reference:
- https://phys.org/news/2021-09-nano-camera-molecular-real-time-chemical.amp
- https://scitechdaily.com/nano-camera-held-together-with-molecular-glue-allows-real-time-monitoring-of-chemical-reactions/amp/
- https://www.sciencedaily.com/releases/2021/09/210902125105.htm














